Freezing bottles – 5 critical considerations
冷冻瓶——5 个关键注意事项
Claus Exenberger
September 25, 2025
Freezing drug substances to ultra-cold temperatures is a standard step in biopharmaceutical manufacturing. Yet, beyond simply cooling, it’s the critical quality attributes (CQA), like product safety, consistency, and process efficiency, that truly define success in biopharma. When preparing to freeze drug substances in sterile bottles, it’s essential to weigh several key factors. These considerations help ensure the freezing process not only preserves product integrity but also supports reliable and productive operations.
将原料药冷冻至超低温是生物制药生产的标准步骤。然而,除了简单的冷却之外,关键质量属性 (CQA),例如产品安全性、一致性和工艺效率,才是真正决定生物制药成功的关键。在准备将原料药冷冻于无菌瓶中时,必须权衡几个关键因素。这些考虑因素有助于确保冷冻过程不仅能保持产品完整性,还能支持可靠且高效的操作。
1. Secure product quality with controlled freezing通过控制冷冻来确保产品质量
Controlled and uniform freezing is essential to avoid cryoconcentration – a phenomenon where solutes concentrate unevenly due to ice formation from the outside in. This can lead to protein denaturation and pH shifts, which ultimately impact drug stability and efficacy. Studies show that the “volcano effect" in bottles, where solutes are pushed to the center and top, can cause significant concentration gradients. Manufacturers can minimize these risks by controlling the freezing rate and temperature profile throughout the freezing process including the solidification phase. Product integrity stems from a controlled freezing which can be adapted depending on the specifications of the drug substance, such as cell-based or mAbs.
受控且均匀的冷冻对于避免低温浓缩至关重要——低温浓缩是一种溶质由于从外部向内结冰而浓缩不均匀的现象。这会导致蛋白质变性和 pH 值变化,最终影响药物的稳定性和疗效。研究表明,瓶中的“火山效应"(溶质被推到中心和顶部)会导致明显的浓度梯度。制造商可以通过控制整个冷冻过程(包括凝固阶段)的冷冻速率和温度曲线来最大限度地降低这些风险。产品完整性源于受控冷冻,可根据药物物质(例如基于细胞或单克隆抗体)的规格进行调整。
What we learnt about controlled bottle freezing
我们对受控瓶冷冻的了解

Figure 1: Visualization of the volcano effect in bottles occurring in uncontrolled freezing, compared to consistent distribution in controlled freezing. 图 1:不受控冷冻时瓶子中火山效应的可视化,与受控冷冻时的一致分布进行比较。
2. Protect bottles for container closure integrity保护瓶子以确保容器密封的完整性
When drug substances or other raw materials, such as buffer or media, are frozen in bottles, the single-use containers must maintain their integrity. Even though they’re called rigid containers, there are vulnerable spots, particularly when the single-use container and assemblies become more brittle under sub-zero conditions.
当药物或其他原材料(例如缓冲液或培养基)在瓶中冷冻时,一次性容器必须保持其完整性。尽管它们被称为硬质容器,但也存在脆弱点,尤其是在零下环境下,一次性容器和组件会变得更脆。
To prevent leakage or contamination, bottle integrity can remain secure when the assemblies are protected during cold chain handling. Bottle RoSS is the equivalent of the RoSS® shell, which is the protective shell for single-use bags. With Bottle RoSS the tubing on bottles is protected at glass-like sub-zero temperatures with the help of a soft 3D foam which hardens at frozen state.
为了防止泄漏或污染,在冷链处理过程中,如果组件受到保护,瓶子的完整性就能得到保障。Bottle RoSS 相当于 RoSS® 外壳,后者是一次性包装袋的保护壳。Bottle RoSS 采用了柔软的 3D 泡沫,在冷冻状态下会变硬,从而保护瓶子上的管子免受零下温度的侵蚀。
More about Bottle RoSS
了解更多关于 Bottle RoSS 的信息
3. Static or blast freezing?静态冷冻还是速冻?
In general, the choice between static and blast freezing for cooling drug substances in bottles depends on the container format and the sensitivity of the drug substances.
一般来说,选择静态冷冻还是速冻来冷却瓶装药物取决于容器的规格和药物的敏感度。
Static freezers are meant for storing drug substances at low temperatures, not for freezing them. Using them to freeze bottles can lead to issues like cryoconcentration and product degradation. Even if the freezing time is similar to other methods for small bottles, the lack of control during the critical phase transition still negatively impacts product quality.
静态冷冻机用于低温储存药物,而非冷冻。使用静态冷冻机冷冻瓶装药物可能会导致低温浓缩和产品降解等问题。即使冷冻时间与其他小瓶冷冻方法相似,关键相变期间的控制不足仍会对产品质量产生负面影响。
In contrast, blast freezers with high-speed air convection technology are more effective at rapidly and uniformly freezing drug substances. This technology is particularly effective for biologics stored in bulky containers, such as bottles, as it minimizes thermal gradients and preserves product quality.
相比之下,采用高速空气对流技术的速冻机能够更有效地快速均匀地冷冻药物。该技术尤其适用于储存在大型容器(例如瓶装)中的生物制剂,因为它可以最大限度地减少热梯度并保持产品质量。

4. Role of bottle types and sizes瓶子类型和尺寸的作用
Not all bottles are created equal. Manufacturers can choose among bottles with different types, geometry and size of the bottle, which may impact on freezing efficiency and uniformity. HDPE and PETG bottles have different features affecting the following fluid and cold chain management processes. Same with the sizes that can range up to 20L bottles.
并非所有瓶子都是一样的。制造商可以选择不同类型、几何形状和尺寸的瓶子,这可能会影响冷冻效率和均匀性。HDPE 和 PETG 瓶具有不同的特性,会影响以下流体和冷链管理流程。容量高达 20L 的瓶子也是如此。
Overall, rigid bottles allow for optimal airflow in blast freezers, supporting consistent freezing profiles. Larger containers may require customized airflow systems to ensure uniform temperature distribution.
总体而言,硬质瓶可在速冻机中实现最佳气流,从而支持一致的冷冻曲线。较大的容器可能需要定制的气流系统,以确保均匀的温度分布。
5. Think end-to-end for closed system efficiency端到端思考,提高封闭系统的效率
Look left and right before freezing bottles. An efficient and safe freezing process requires an aseptic closed system, beginning with the aseptic filling and filtration stages. This continues through cold chain storage and shipping with a controlled thawing process. Integrating closed systems minimizes contamination risks, streamlines workflows, and ensures that drug substances remain protected at every stage. This end-to-end strategy meets GMP-relevant quality standards, including Annex 1, and operational efficiency, making it the gold standard for working with bottles in biopharma.
冷冻瓶子前务必仔细检查。高效安全的冷冻流程需要无菌密闭系统,从无菌分装和过滤阶段开始,一直到冷链储存和运输,并采用受控解冻流程。集成密闭系统可最大限度地降低污染风险,简化工作流程,并确保药物在每个阶段都得到保护。这种端到端策略符合 GMP 相关质量标准(包括附录 1)和运营效率,使其成为生物制药行业使用瓶子的黄金标准。
Interview: Trends with Single-Use Bottles in Bioprocessing – Single Use Support
访谈:生物加工中一次性瓶的趋势——一次性支持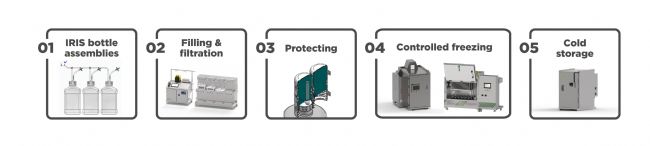
Mastering filling & freezing of bottles with Single Use Support利用一次性使用支持掌握瓶子的分装和冷冻
Uncontrolled bottle freezing processes often struggle to scale, leading to inefficiencies and risks for critical drug substances. With RoSS.BLST, Single Use Support addresses these pain points by enabling seamless transitions from clinical to commercial production – without compromise.
不受控制的瓶装冷冻工艺通常难以规模化,导致关键药物效率低下并带来风险。借助 RoSS.BLST,Single Use Support 解决了这些痛点,实现了从临床到商业生产的无缝过渡,且丝毫不受影响。
Single Use Support’s end-to-end fluid management and cold chain solutions deliver scalable, aseptically closed processes for advanced fluid and cold chain management of bottles, ensuring product safety and regulatory compliance at every step. As the only provider offering fully integrated handling, filling and cooling critical liquids in bottles, Single Use Support empowers biopharma manufacturers to streamline fluid and cold chain management with bottles with compatibility, safety and efficiency.
Single Use Support 的端到端流体管理和冷链解决方案为先进的瓶装流体和冷链管理提供可扩展、无菌封闭的流程,确保每一步的产品安全和合规性。作为一家提供全集成瓶装关键液体处理、分装和冷却的供应商,Single Use Support 使生物制药制造商能够使用兼具兼容性、安全性和效率的瓶子来简化流体和冷链管理。