The situation 情况
The bottle: Friend & foe in bioprocessing 瓶子:生物加工中的朋友和敌人
Despite the growing shift toward single-use bags, sterile bottles remain a staple in many biopharmaceutical workflows, such as
尽管人们越来越多地转向使用一次性袋子,但无菌瓶仍然是许多生物制药工作流程中的主要组成部分,例如
Early-stage development 早期开发
Small-volume fills 小容量分装
Stability studies 稳定性研究
Buffer, media, solution addition 缓冲液、培养基、溶液添加
Their rigid structure and compatibility with existing lab infrastructure make them a reliable choice in specific scenarios.
它们的坚固结构和与现有实验室基础设施的兼容性使其成为特定场景中的可靠选择。
Bottles present limitations in modern bioprocessing: Fluid management utilizing bottles is less scalable and difficult to automate. Additionally, bottles pose contamination risks due to open handling while also delivering inconsistent freezing results.
在现代生物工艺中,瓶子的使用存在诸多限制:使用瓶子进行液体管理的可扩展性较差,且难以实现自动化。此外,瓶子由于采用开放式操作,存在污染风险,并且会导致冷冻结果不一致。
However, advanced bottle filling technologies can turn this “foe" into a trusted “friend", tackling these challenges with precision and sterility.
然而,先进的瓶装技术可以将这个“敌人"变成值得信赖的“朋友",以精准和无菌的方式应对这些挑战。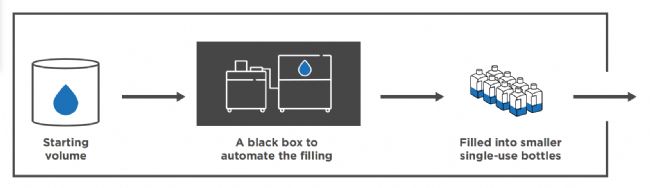
Case: Media filling into single-use bottles and 3D bags 案例:填充至一次性瓶和3D袋中
A biopharmaceutical manufacturer based in Latin America specializes in the production of liveattenuated vaccines. The upstream processing (USP) of these vaccines focuses on highdensity cell cultures, which are later used for virus activation and amplification. For these bioprocessing steps in cell expansion and virus propagation it is required to use media and buffer solutions.
一家位于拉丁美洲的生物制药制造商专门生产减毒活疫苗。这些疫苗的上游加工(USP)侧重于培养高密度细胞,随后用于病毒的激活和扩增。在细胞扩增和病毒增殖的这些生物加工步骤中,需要使用培养基和缓冲液。
To maintain sterility and process flexibility, the company uses single-use bottles and 3D bags for aseptic media filling. The process accommodates a wide range of container formats – from bottles of 30mL to 2000mL, to large 3D single-use bags ranging from 50L to 200L.
为了保持无菌性和工艺灵活性,该公司采用一次性瓶和3D袋进行无菌培养基分装。该工艺适用于各种容器规格——从30毫升到2000毫升的瓶子,到50升到200升的大型3D一次性袋。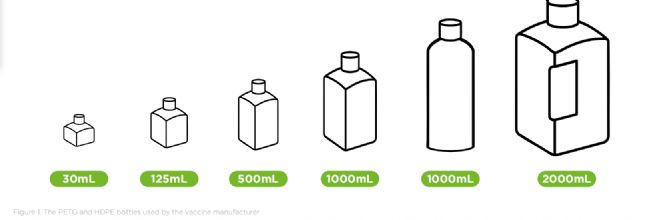
The challenge 挑战
Complex media filling operations 复杂的培养基分装操作
Due to the large variety and quantity of containers – up to 20 single-use bottles and 3D bags per batch – the manufacturer faces significant operational challenges.
由于容器种类繁多、数量庞大——每批次多达20个一次性瓶和3D包装袋——制造商面临着巨大的运营挑战。
This complexity reduces efficiency and limits flexibility, particularly when adapting to varying batch sizes or container formats. Manual filling in a biosafety cabinet or isolator carries significant safety risks, raising the chance of contamination and jeopardizing product sterility. Together, these factors impede scalability and process robustness—making automation and closed-system solutions ever more essential.
这种复杂性降低了效率,并限制了灵活性,尤其是在适应不同的批次大小或容器规格时。在生物安全柜或隔离器中手动分装存在重大安全风险,会增加污染的可能性,并危及产品的无菌性。这些因素共同阻碍了可扩展性和工艺的稳健性——这使得自动化和封闭系统解决方案变得更加重要。

The solution 解决方案
Making bottle filling a closed system 使瓶子分装成为一个封闭的系统
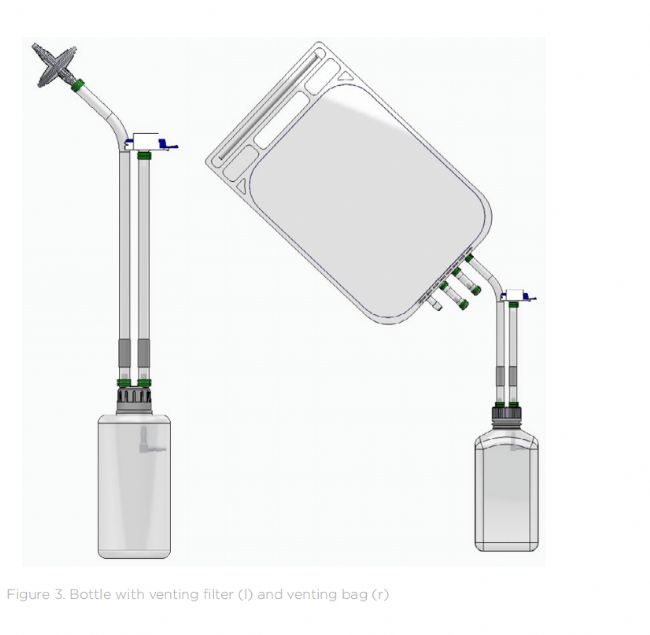
In aseptic manufacturing, converting bottle filling into a fully closed system is a key step in preserving product sterility across the entire fluid management process. This is achieved by using bottle caps equipped with sterile venting filters or venting bags that allow for pressure equalization during automated filling without exposing the product to the environment. Customizable singleuse bottle assemblies can be specifically designed to integrate with automated bottle filling systems, eliminating the need for manual intervention. The result: reduced contamination risk, assured GMP compliance (including Annex 1), and less reliance on high-grade cleanroom facilities.
在无菌生产中,将瓶装分装系统转换为全封闭系统是在整个流体管理过程中保持产品无菌的关键步骤。这可以通过使用配备无菌排气过滤器或排气袋的瓶盖来实现,这些过滤器或排气袋可以在自动分装过程中保持压力平衡,而不会将产品暴露在环境中。可定制的一次性瓶组件可以专门设计用于与自动瓶装分装系统集成,从而无需人工干预。结果:降低污染风险,确保符合GMP标准(包括附录1),并减少对高级洁净室设施的依赖。
One size fills all: Automated & precise bottle filling with RoSS.FILL 一种尺寸适合所有情况:RoSS.FILL 自动精准分装
Accommodating a wide range of container types and volumes, RoSS.FILL Bottle delivers a highly flexible and scalable solution for automated filling of both bottles and 3D single-use bags. Engineered for manufacturing cleanrooms, the system supports closed-system filling, ensuring precise, pressure-balanced operation and batch consistency for GMPcompliant dispensing.
RoSS.FILL Bottle 适用于各种类型和容量的容器,为瓶子和 3D 一次性包装袋的自动分装提供了高度灵活且可扩展的解决方案。该系统专为洁净室制造而设计,支持封闭式系统分装,确保精准、压力平衡的运行和批次一致性,从而符合 GMP 要求。
Its modular design enables seamless setup changes, making it ideal for multiproduct facilities and dynamic production needs. The vaccine manufacturer was now able to use a setup with different racks connected to a single control unit to fill large volumes of media and buffer solution. This made it possible to fill both bottles and bags using the same system, tailored to the required packaging format (see figure 4).
其模块化设计可实现无缝设置更改,使其成为多产品设施和动态生产需求的理想选择。现在,该疫苗制造商能够使用连接到单个控制单元的不同支架的装置来分装大量培养基和缓冲液。这使得能够使用同一系统同时分装瓶子和包装袋,并根据所需的包装规格进行定制(见图 4)。

The result 结果
Being able to fill bottles of varying sizes and volumes has markedly improved operational efficiency over manual methods. Specifically, the vaccine manufacturer reported the following benefits for bottle filling:
与手动方法相比,能够分装不同尺寸和容量的瓶子显著提高了操作效率。具体来说,疫苗制造商报告了瓶子分装的以下优势:
More than 3 times faster filling 分装速度提高 3 倍以上
Including preparation, filling, and documentation in a sterile environment, the manual process averages 5 hours, while automation completes the task in just over 1 hour. Dispensing 150 liters into 96 single-use 2-liter bottles was thus approximately 70% faster with RoSS.FILL than with manual handling.
包括在无菌环境中的准备、分装和记录,手动流程平均耗时 5 小时,而自动化流程仅需 1 个多小时即可完成。因此,使用 RoSS.FILL 将 150 升液体分装到 96 个 2 升一次性瓶中,比手动操作快约 70%。
Only 1 operator needed 仅需 1 名操作员
Including material handing and quality control, a standard manual filling process requires two to three operators, whereas automated filling machines can be operated by one person. All documentation is fully recorded in accordance with 21 CFR Part 11 requirements.
包括物料处理和质量控制在内,标准的手动分装流程需要两到三名操作员,而自动分装机只需一人即可操作。所有文件均按照21 CFR Part 11的要求完整记录。
The time saved in filling bottles and bags in parallel increases significantly when relying on automatic filling. This is especially true with modular systems, which enable both containers to be filled using one system.
采用自动分装技术,可显著节省同时分装瓶子和袋子的时间。模块化系统尤其如此,它允许使用一个系统同时分装两个容器。

Additional benefits with closed bottle filler 封闭式分装机的额外优势
Aseptically closed system: Using a closed system significantly reduces the risk of human error and exposure to bioburden, especially when working with sensitive biologics, by minimizing open transfers and operator interaction. 无菌密闭系统:使用密闭系统可显著降低人为失误和生物负载暴露的风险,尤其是在处理敏感生物制剂时,因为它可以最大限度地减少开放式转移和操作员互动。
Accuracy: Filling processes can be tailored to meet strict customer requirements – such as ±100g for large bags an ±10g for bottles – ensuring consistent and compliant output. 准确性:分装流程可根据严格的客户要求进行定制,例如大袋±100克,瓶装±10克,从而确保输出的一致性和合规性。
Recovery rate: The system enables maximum recovery rates of any drug substance or drug product, minimizing waste and maximizing yield. 回收率:该系统可提高任何原料药或药品的回收率,从而最大限度地减少浪费并提高产量。
GMP compliance: Integrated software solutions that are fully compliant with 21 CFR Part 11, cGMP, and Annex 1 regulations allow supervisor-level control. These features support traceability, audit readiness, and overall process reliability. Together, they contribute to a robust and quality-driven manufacturing environment. 符合GMP标准:集成的软件解决方案符合21 CFR第11部分、cGMP和附录1法规,可实现主管级控制。这些功能支持可追溯性、审计准备度和整体流程可靠性。它们共同构成了强大且以质量为导向的生产环境。
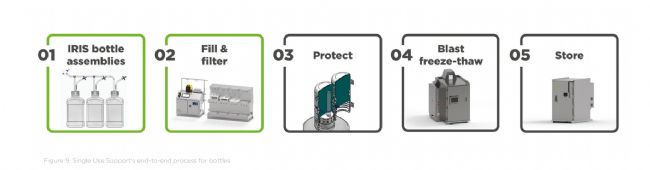
Integrated into the end-to-end bottle process: Like with single-use bags, Single Use Support offers complete solutions for the entire fluid and cold chain management of bottles. These include: 集成到端到端瓶装流程中:与一次性包装袋一样,一次性支持系统为整个瓶装液体和冷链管理提供完整的解决方案。这些解决方案包括:
IRIS Bottle Assemblies: Single-use tubing and manifold assemblies developed for your fluid transfer processes in biopharma processes. IRIS 瓶组件:专为生物制药工艺中的流体输送流程而开发的一次性管路和歧管组件。
Bottle RoSS®: Protects tubing assemblies and bottle caps during freezing to ensure product integrity. RoSS® 瓶:在冷冻过程中保护管路组件和瓶盖,确保产品完整性。
RoSS.BLST: GMP-compliant system for blast freezing & thawing of drug substances – suitable for any primary packaging, including bottles. RoSS.BLST:符合 GMP 标准的药物速冻和解冻系统,适用于任何初级包装,包括瓶装。
lRoSS.ULTF: Ultra-low temperature storage freezer for frozen drug substances in any primary packaging, maintaining stable temperatures down to -80°C
RoSS.ULTF:超低温冷冻柜,适用于任何初级包装的冷冻药物,可在低至 -80°C 的温度下保持稳定温度。